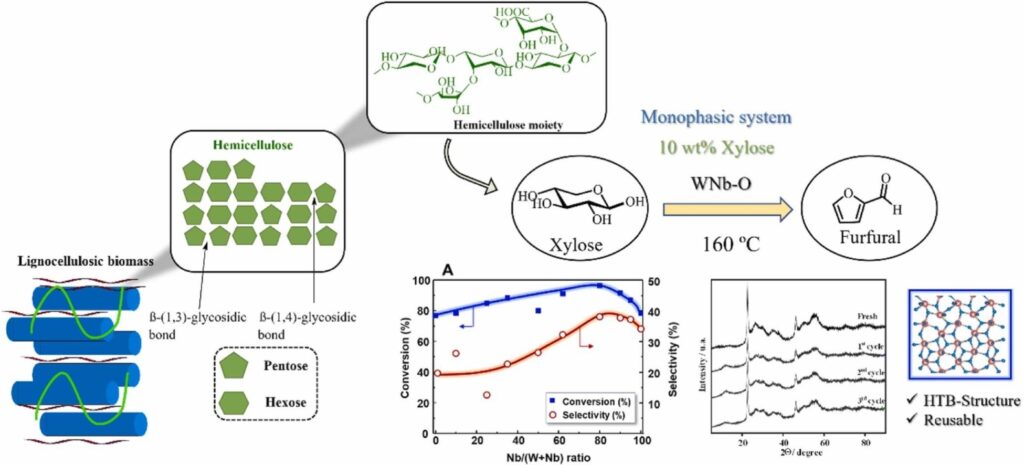
Dehydration of xylose to furfural catalyzed by highly stable W-Nb-O mixed oxides Bronzes
Appl. Catal. A: Gen. 2025, 674, 120912
https://doi.org/10.1016/j.apcata.2025.120467
Abstract
In this work, the dehydration reaction of xylose (a representative C5 sugar) to furfural has been studied over WNb-O mixed oxides presenting hexagonal or pseudo-crystalline Bronze-type structure with controlled textural and acid properties, and also high stability under reaction conditions. Thus, a series of W-Nb-O metal oxides with different W/Nb ratios have been prepared hydrothermally, characterized by several physicochemical techniques and tested in autoclave-type reactors in liquid phase. Their influence on the catalytic activity has been assessed by tuning the Brønsted/Lewis acid properties of W-Nb-O materials. Interestingly, optimal catalytic results for the dehydration of xylose to furfural in monophasic system (water/ethanol mixture) were achieved for W0.20Nb0.80Ox catalyst, with a furfural selectivity comparable to that of sulfuric acid industrial catalyst. Furfural productivity (0.683 gFur·gCat-1·h-1) attained with this W-Nb-O catalyst was higher than that calculated for most relevant and recently reported solid acid catalysts working in both mono- and bi-phasic systems, respectively. Catalytic activity of W-Nb-O material remained practically unchanged after three consecutive reuses, while structure and chemical composition of material were maintained unaltered either after three catalytic cycles or after 14 days experiment carried out under optimal reaction conditions. Reactivity results have been explained on the combination of both Brønsted and Lewis acid sites as a result of the different incorporation of niobium into the bronze structure.
Synthesis of Sulfated Sn–Zr Mesoporous Catalysts for theSelective Dehydration of Hexose-Type Monosaccharides
Chem. Open 2025, 14, e202400480
https://doi.org/10.1002/open.202400480
Abstract
Synthesis of combined Brønsted and Lewis acid sites in SBA-15 by Zrand Sn incorporation onto the mesoporous support, followed by asulfation procedure to get enhanced acid catalysts for fructose-to-5-hydroxymethylfurfural (HMF) selective dehydration, is here studied.Different preparation methods are used to attain the SulfatedSn–Zr–SBA-15 material with adequate combination of Brönstedand Lewis acidity. Samples are characterized by using different ana-lytical and spectroscopic techniques, results indicating a correlationbetween the preparation procedure and the acid characteristics ofthe materials. The latter are evaluated in the catalytic conversion ofconcentrated fructose solutions (30 wt%) into HMF in a biphasicsystem (water þ iso-butylketone/2-butanol). The most efficient cat-alyst, SO4/Sn–Zr–SBA-15 (Zr/Sn molar ratio = 1.98), yields ≥ 70%HMF at a substrate conversion of ≈90% under optimal reaction con-ditions (150 °C for 120 min.), these results being particularly relevantwhen considering the higher initial fructose concentrations hereused compared to those typically employed in previous reports.This catalyst exhibits a higher ratio of Bronsted/Lewis (B//L) acid sitesof moderate strength and a lower density of acid sites compared tothe other catalytic samples evaluated, mainly due to the presence ofwell-dispersed tin and zirconium species, as well as to the enhancedacidic properties provided by sulfate groups in the material.